Safety Standards for Regenerative Medicine: Exosomes in Riyadh 2026
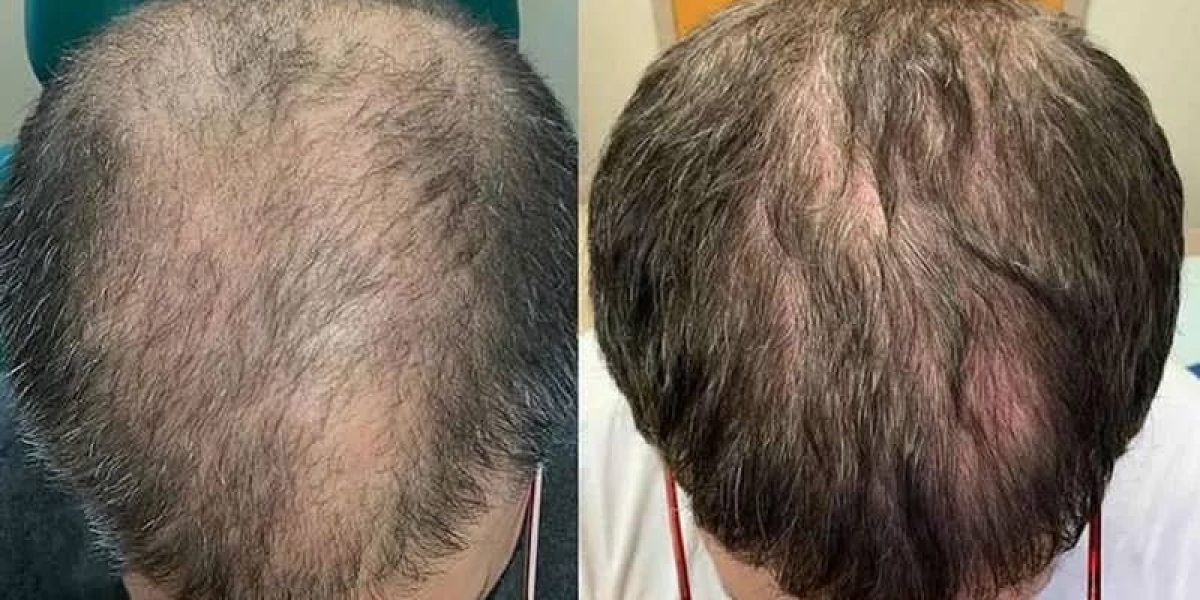
The rapid expansion of the Saudi healthcare sector under Vision 2030 has placed the capital at the forefront of biotechnological safety. Treating hair loss with exosomes in Riyadh(تساقط الشعر بالإكسوسومات في الرياض) is governed by a rigorous regulatory framework in 2026, ensuring that the "high-tech promise" of regeneration is matched by "high-standard" patient protection. As regenerative medicine moves from specialized research labs to mainstream aesthetic clinics, the Saudi Food and Drug Authority (SFDA) has established comprehensive guidelines for the handling, purification, and administration of extracellular vesicles. For the patient, these standards provide a critical layer of assurance, distinguishing medical-grade restoration from unverified commercial trends and ensuring that every session is both biologically potent and clinically secure.
The Regulatory Pillars of the SFDA (2026)
In 2026, the SFDA classifies exosomes as "Biological Medicinal Products." This classification subjects them to the same level of scrutiny as high-end pharmaceuticals and vaccines.
Source Traceability: Every exosome batch used in Riyadh must have a "Certificate of Origin." This ensures the mesenchymal stem cells (MSCs) used for harvesting are ethically sourced and screened for infectious diseases, genetic abnormalities, and contaminants.
Standardized Purity (Nano-Filtration): To be approved for clinical use, exosome solutions must undergo rigorous purification processes, such as tangential flow filtration (TFF) and ultracentrifugation. These methods remove cellular debris and non-essential proteins, leaving only the pure, signaling vesicles required for hair growth.
Sterility and Endotoxin Testing: Before any product reaches a clinic in Al-Qassim or Riyadh, it must pass a "Batch Release" test. This confirms the solution is sterile and free from endotoxins, which are bacterial by-products that could cause inflammation or adverse scalp reactions.
Clinical Protocols: Safe Administration in Riyadh
Safety in 2026 extends beyond the product itself to the environment in which it is administered. Riyadh’s leading centers adhere to strict "Point-of-Care" safety protocols.
Professional Certification: Exosome therapy in the capital is strictly limited to licensed dermatologists or plastic surgeons who have undergone specialized training in regenerative signaling. This prevents the "misapplication" of the technology by untrained personnel.
The Microneedling vs. Injection Standard: While research into direct injections continues, the 2026 safety gold standard in Riyadh often favors microneedling-assisted delivery. By creating micro-channels, clinicians allow the nano-vesicles to reach the dermis without the deeper risks associated with high-volume subcutaneous injections.
Adverse Event Monitoring: Approved clinics are required to participate in a "Pharmacovigilance" system, reporting any unusual patient reactions to a centralized SFDA database. This real-time monitoring ensures that the safety profile of exosomes remains transparent and robust across the Kingdom.
Safety Comparison: Exosomes vs. Traditional Methods
| Feature | Hair Transplant Surgery | Exosome Therapy (2026 Safety) |
| Invasiveness | High (Incision/Anesthesia). | Minimal (Micro-perforation). |
| Risk of Infection | Moderate (Open wounds). | Negligible (Sterile, closed system). |
| Immune Response | Risk of "Shock Loss." | Biocompatible (Cell-free). |
| Recovery Side Effects | Swelling and scabbing. | Mild redness (fades in $<24$ hours). |
| Regulation Status | Well-established surgical laws. | Strict Biological Product Laws (SFDA). |
Understanding the "Cell-Free" Safety Advantage
A primary reason for the high safety rating of exosomes in 2026 is their "cell-free" nature.
No DNA Transfer: Exosomes carry RNA and proteins but do not contain a cell nucleus. This means they cannot replicate on their own or integrate into the patient's DNA, eliminating the risk of "malignant transformation" that was a concern with early-generation live stem cell therapies.
Low Immunogenicity: Because they lack the surface proteins that trigger immune rejection (MHC complexes), exosomes are generally "invisible" to the patient's immune system. This makes them significantly safer for a wide range of patients, including those with sensitive skin or mild autoimmune predispositions.
Conclusion
The safety of exosome therapy in Riyadh is a reflection of the Kingdom's commitment to world-class medical excellence. By 2026, the combination of SFDA oversight, standardized lab purification, and expert clinical administration has made treating hair loss with exosomes in Riyadh one of the most secure aesthetic procedures available. When you choose an approved clinic in the capital, you aren't just choosing a path to thicker hair; you are choosing a treatment backed by the highest standards of modern regenerative science.